Cynata Therapeutics
Cell Therapy
Background
Cynata is an Australian Stock Exchange-listed biotechnology company whose founders invented the Cymerus™ technology, a proprietary process of generating cell-based products from mesenchymal angioblasts (MCAs), which in turn are derived from induced pluripotent stem cells (iPSCs). The cornerstone of Cynata’s intellectual property is the ability to turn iPSCs into clinical grade mesenchymal stem cells (MSCs) in a consistent, reproducible way. As a result, Cynata can produce an essentially limitless number of MSCs from the same starting material derived from a single donor. Furthermore, it means that Cynata does not need to excessively expand MSCs in culture in order to produce large numbers of doses.
In early 2015, Cynata had successfully transferred the Cymerus™ technology to a GMP environment, allowing the company to move the lead product, CYP-001, into the clinic. Graft versus host disease (GvHD) was selected as the initial indication to demonstrate proof of concept.
Objective
In mid-2015, Cynata contacted Plexus Ventures for assistance in securing partners for the Cymerus™ technology and the preclinical compound, CYP-001.
Process
Plexus Ventures generated a non-confidential and confidential information memorandum, together with Cynata, to effectively describe the benefits of the technology to potential partners. In January 2016, Plexus Ventures initiated contact with 70 biotech and pharmaceutical companies selected for their known pursuit of cell-based therapies. One of the interested companies, FUJIFILM, informed Plexus Ventures they had been in prior discussions on the Cymerus™ technology through their US-based subsidiary, Cellular Dynamics Inc. (CDI). The following month, Plexus Ventures arranged a meeting between Cynata and FUJIFILM in Tokyo to elevate the discussions to corporate level and confirm strategic interest in this therapeutic product.
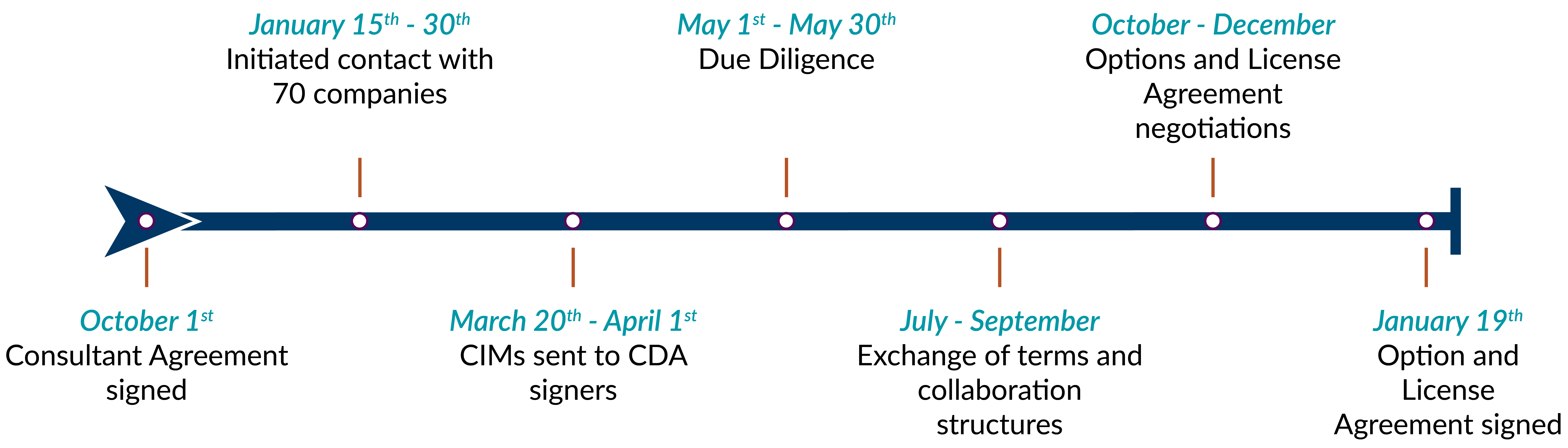
Outcome
Plexus Ventures leveraged its on-the-ground presence in Tokyo and familiarity with decision-making process in Japanese companies to ensure continued progress towards reaching final agreement of terms. An option agreement was announced in January 2017, 12 months from initiation of the Plexus contact program. Following success of the Phase I trial in GvHD, in September 2019, FUJIFILM exercised the option to an exclusive, worldwide license to develop and commercialize CYP-001 for prevention and treatment of GvHD. The exercise served to further validate Cynata’s technology platform and the significant commercial opportunity of CYP-001.
