Cinfa
Biosimilars
Background
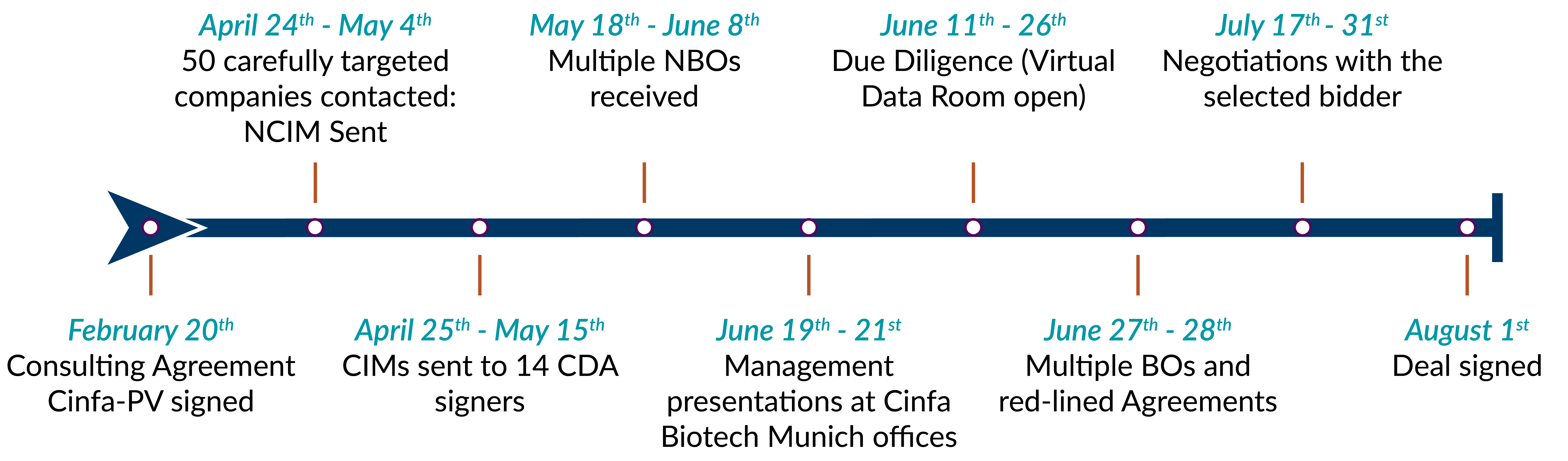
In late 2017, Cinfa Group, the parent of Spain’s largest generic manufacturer, Laboratorios Cinfa S.A., identified its subsidiary, Cinfa Biotech (Munich, Germany), a biosimilar development company, as non-strategic to the Group’s future. Cinfa Biotech had cost-effectively developed Pelmeg®, a biosimilar of Neulasta (pegfilgrastim) and had convinced regulatory authorities Phase I data would be sufficient for regulatory approval. However, Cinfa Group did not have the requisite biosimilar commercial capabilities to successfully market the product.
Objective
In late 2017 — reversing a prior decision to invest heavily in biosimilar development — the Board of Directors of Cinfa Group approved a strategic recommendation to invest those resources in support of its small molecule and specialty generic products and in its consumer healthcare product lines. This decision would deprive Cinfa Biotech of resources required for future biosimilar development, despite the outstanding performance. Cinfa, which had entertained initial conversations with potential licensing partners, decided to divest its 100% ownership stake in Cinfa Biotech to either a strategic pharmaceutical player or a private equity investor. To prepare for a timely commercial launch, the transaction needed to be closed quickly as the European Medicines Agency (“EMA”) would likely grant Market Authorization for Pelmeg® within 6 to 9 months.
Process
In March, 2018, Cinfa appointed Plexus Ventures — already well-known for the completion of two high profile biosimilar out-licensing transactions — to divest Cinfa Biotech. The Plexus team reached out to 49 companies, 14 of whom received the detailed confidential information package. Plexus received multiple Non-Binding and, later, Binding Offers from both pharmaceutical companies and private equity funds.
Outcome
In mid-September, 2018, the Committee for Medicinal Products for Human Use (“CHMP”) gave recommendws Pelmeg® for approval to EMA. On October 11th 2018, Mundipharma acquired 100% ownership of Cinfa Biotech, thus adding a ready-to-launch product to its existing biosimilars portfolio, comprising Remsima® and Truxima®. Pelmeg® was launched in January, 2019 and the product has been successfully marketed in countries throughout Europe.
